Hello young scientists!
Welcome to Sciences4Kids where we learn about all kinds of cool science topics. Today, we are going to learn about separation techniques which are special methods that scientists use in separating different substances from each other. These techniques are really important in different science fields, like chemistry, biology, and environmental science.
https://sciences4kids.science.blog/2023/01/02/the-chemistry-of-everyday-life/
There are several different types of separation techniques in science and that is what we will be talking about today.. So, let’s get started!
Distillation
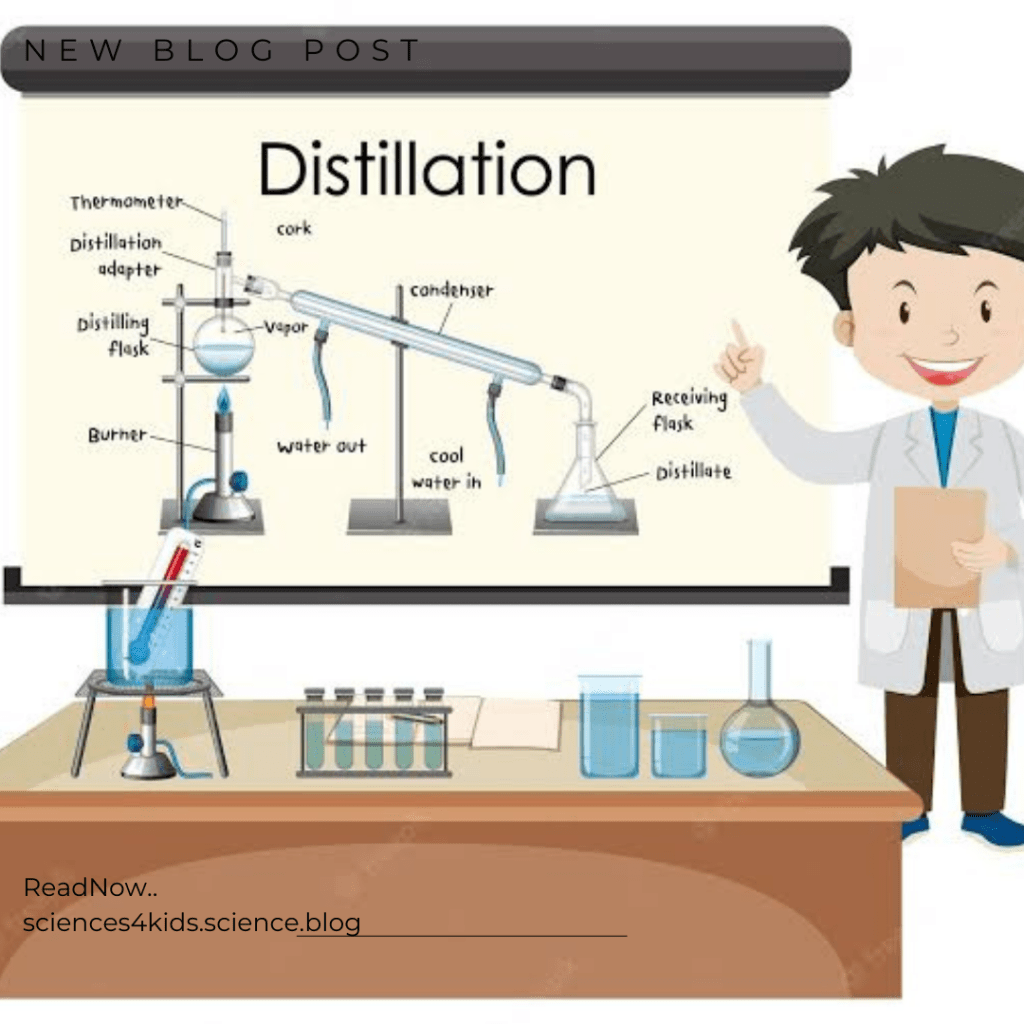
Distillation is the separation technique that is used when separating liquids based on their boiling points. You might be wondering, “What is a boiling point?” Well, a boiling point is the temperature at which liquid turns into a gas during or after the heating process. Different liquids have different boiling points, which means that they will turn into gas at different temperatures. Distillation takes advantage of this by heating up a mixture of two liquids until they turn into gases. These gases are then cooled back down and turned back into liquids. The two liquids will then be separated because they now have different boiling points.
There are two main types of distillation and they are: simple distillation and fractional distillation.
Simple Distillation
Simple distillation is used in separating two liquids that have big differences in their boiling points. For example, if you have a mixture that contains water and oil, you could use simple distillation in separating them. Oil has a higher boiling point than water, so it would turn into a gas later. This means that the water would be turned into a gas first, and then the oil would be turned into a gas later.
Fractional Distillation
Fractional distillation is a little more complicated. It is used in separating two liquids that have similar boiling points. For example, if you have a mixture that contains ethanol (a type of alcohol) and water, you could use fractional distillation to separate them. Ethanol and water have very similar boiling points, so it is hard to separate them using simple distillation. Fractional distillation works by heating the mixture up and then cooling it down very slowly. This causes the ethanol and water to separate into two different liquids.
Distillation is a really useful technique because it can be used to purify water. Sometimes, water can contain impurities like dirt or bacteria and by using distillation, we can heat the water up until it turns into a gas, and then cool it back down again. This leaves the impurities behind, so we are left with clean, pure water. Distillation is also used to separate ethanol and water. Ethanol is used in lots of different products, like hand sanitizer and rubbing alcohol.
Filtration
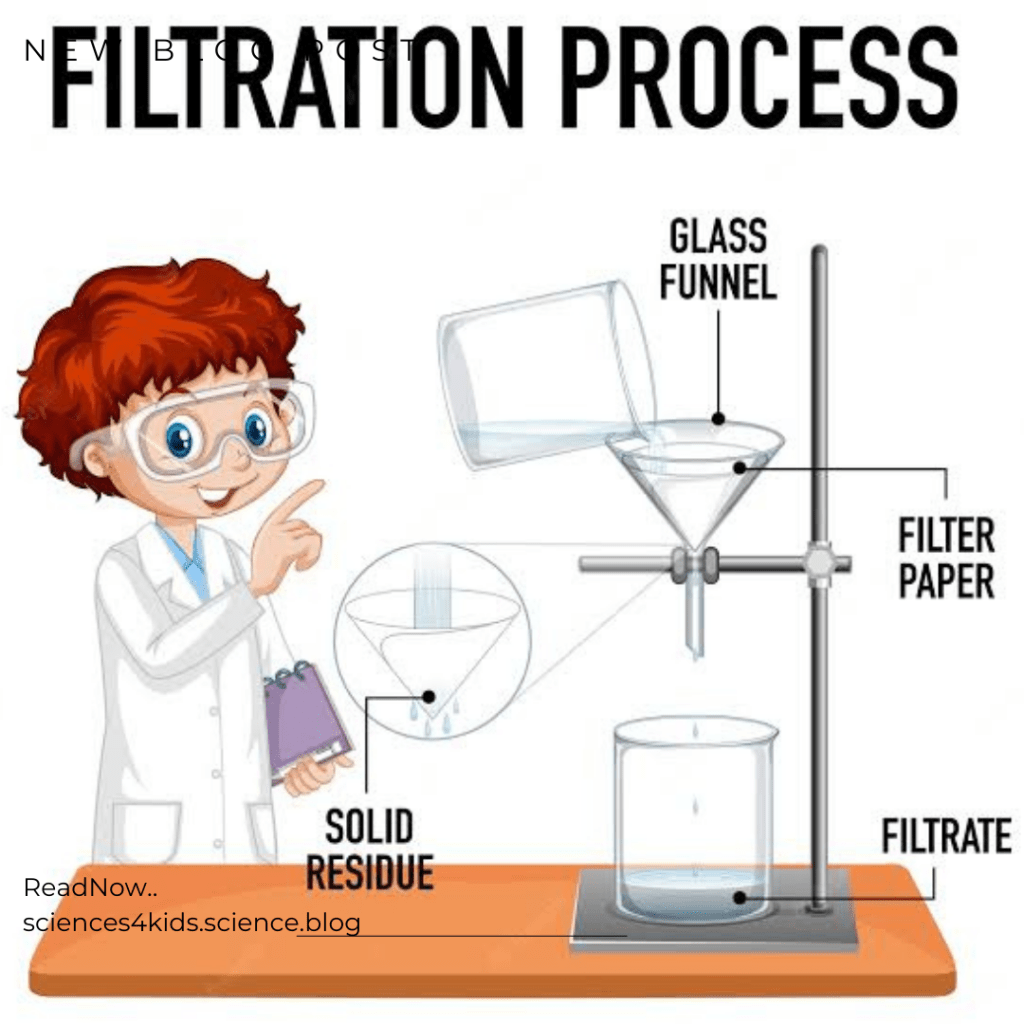
Filtration is a really useful technique because it can be used to remove solid particles from liquids. This is important because sometimes we need to get rid of solid particles that are mixed in with a liquid. For example, if you have a mixture of water and dirt, you could use filtration to separate them. The water would pass through the filter, and the dirt would be left behind.
Filtration is also used to separate mixtures. Sometimes, we have a mixture of two or more substances, and we want to separate them. Filtration can help us do this by using a special filter that only lets one of the substances pass through. For example, if you have a mixture of oil and water, you could use filtration to separate them. The water would pass through the filter, and the oil would be left behind.
There are two main types of filtration and they are the gravity filtration and the vacuum filtration.
Gravity Filtration
Gravity filtration is used when the solid particles are heavier than the liquid. For example, if you have a mixture of water and sand, you could use gravity filtration to separate them. The water would pass through the filter, but the sand would be left behind.
Vacuum Filtration
Vacuum filtration is used when the liquid is heavier than the solid particles. For example, if you have a mixture of oil and water, you could use vacuum filtration to separate them. The water would pass through the filter, but the oil would be left behind.
There are lots of different filters that can be used for filtration, depending on what you are trying to separate. Some common filters include paper filters, cloth filters, and special filters made of materials like glass or ceramic. Scientists use filtration all the time to separate different substances and to purify liquids.
Chromatography
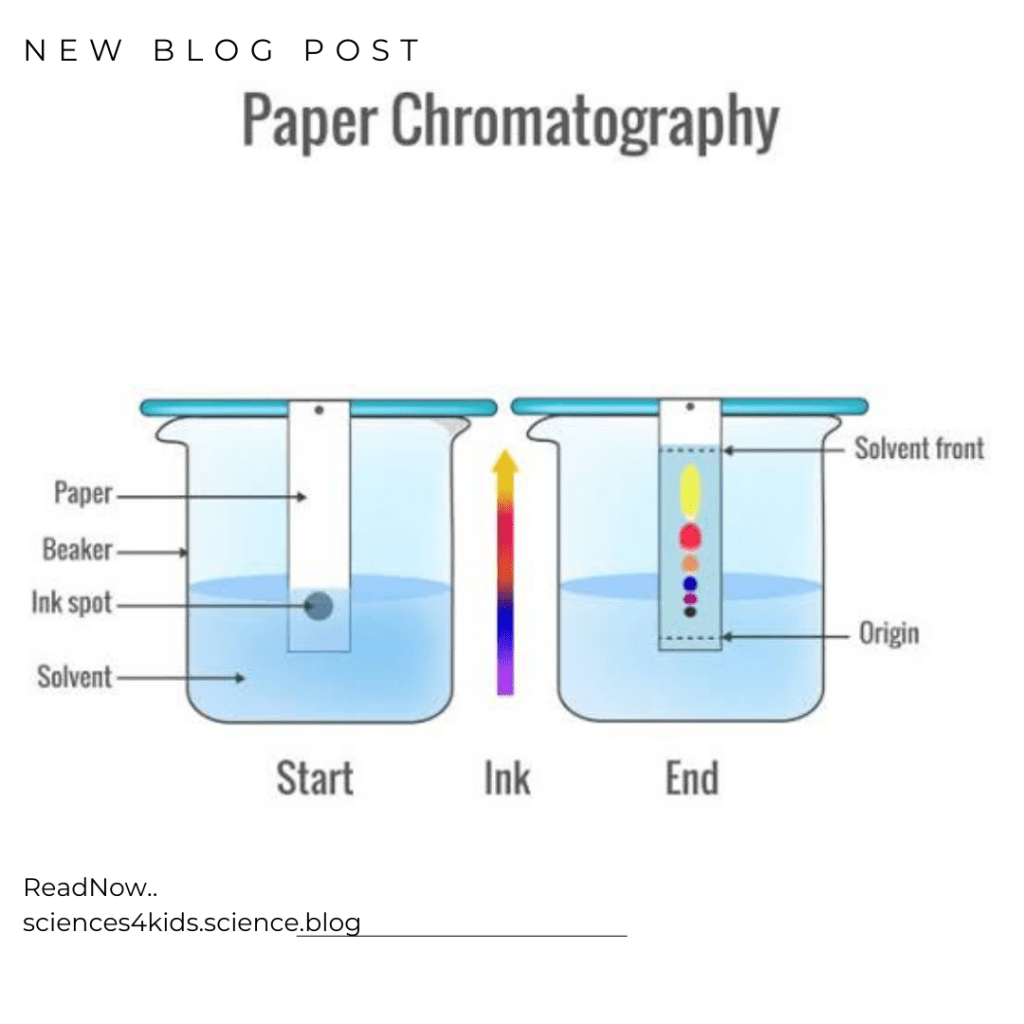
Chromatography is a technique that is used to separate mixtures based on the different properties of the substances in the mixture. When you have a mixture of two or more substances, chromatography can help you separate them. The way it works is by using a special material called a stationary phase, which can be a piece of paper or a column filled with a special substance. The mixture is dissolved in a solvent and then passed through the stationary phase. The different substances in the mixture will move at different rates through the stationary phase because they have different properties.
There are two main types of chromatography: paper chromatography and gas chromatography.
Paper Chromatography
Paper chromatography is used to separate substances that are dissolved in a liquid. For example, if you have a mixture of different colors of ink, you could use paper chromatography to separate them. The different colors of ink would move at different rates through the paper, and so they would be separated.
Gas Chromatography
Gas chromatography is used to separate substances that are gases. For example, if you have a mixture of different gases, you could use gas chromatography to separate them. The different gases would move at different rates through the stationary phase, and so they would be separated.
Chromatography is a really useful technique because it can be used to analyze mixtures. Sometimes, we need to know what is in a mixture, and chromatography can help us find out. Chromatography is also used to purify substances. For example, if you have a mixture of two different substances, you can use chromatography to separate them and then collect one of the substances.
Centrifugation
Centrifugation is a technique that is used to separate substances based on their densities. Density is a measure of how much matter is packed into a certain space. Some substances are more dense than others, which means that they have more matter packed into the same amount of space. Centrifugation takes advantage of this by spinning a mixture around very fast. The heavier substances will move to the bottom of the mixture, while the lighter substances will stay at the top.
There are two main types of centrifugation: sedimentation centrifugation and density gradient centrifugation.
Sedimentation Centrifugation
Sedimentation centrifugation is used to separate substances based on their size. For example, if you have a mixture of small and large particles, you could use sedimentation centrifugation to separate them. The large particles would move to the bottom of the mixture, while the small particles would stay at the top.
Density Gradient Centrifugation
Density gradient centrifugation is used to separate substances based on their density. For example, if you have a mixture of two different substances that have different densities, you could use density gradient centrifugation to separate them. The substance with the higher density would move to the bottom of the mixture, while the substance with the lower density would stay at the top.
Centrifugation is a really useful technique because it can be used to separate cells and subcellular particles. This is important because sometimes we need to study cells or subcellular particles on their own, without anything else mixed in. Centrifugation is also used to purify proteins. Proteins are important molecules that do lots of different jobs in our bodies, and it is important to be able to purify them so that we can study them.
Extraction
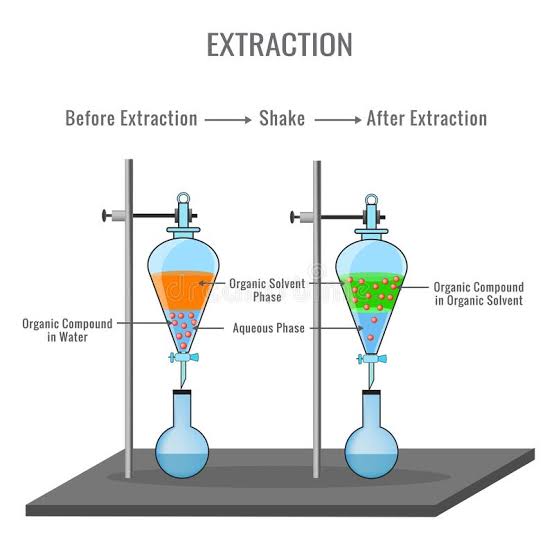
Extraction is a technique that is used to separate a substance from a mixture based on its solubility. Solubility is the ability of a substance to dissolve in a solvent. Different substances will dissolve in different solvents, and so we can use extraction to separate them.
There are two main types of extraction: solvent extraction and solid phase extraction.
Solvent Extraction
Solvent extraction is used to separate substances that are dissolved in a liquid. For example, if you have a mixture of a substance dissolved in water, you could use solvent extraction to separate it. You would need to choose a solvent that the substance will dissolve in, but not the water. Then, you could mix the mixture with the solvent, and the substance would dissolve in the solvent. The solvent and the substance could then be separated from the water.
Solid Phase Extraction
Solid phase extraction is used to separate substances that are present in a solid. For example, if you have a mixture of a substance mixed in with some dirt, you could use solid phase extraction to separate them. You would need to choose a solvent that the substance will dissolve in, and then you would mix the mixture with the solvent. The substance would dissolve in the solvent, and then you could filter the mixture to separate the solvent and the substance from the dirt.
Extraction is a really useful technique because it can be used to purify compounds. Sometimes, we need to remove impurities from a compound so that we can study it or use it. Extraction is also used to isolate natural products. Natural products are substances that are found in nature, like plants or animals. Scientists are interested in studying natural products because they can have special properties that are useful for medicine or other purposes.
Precipitation
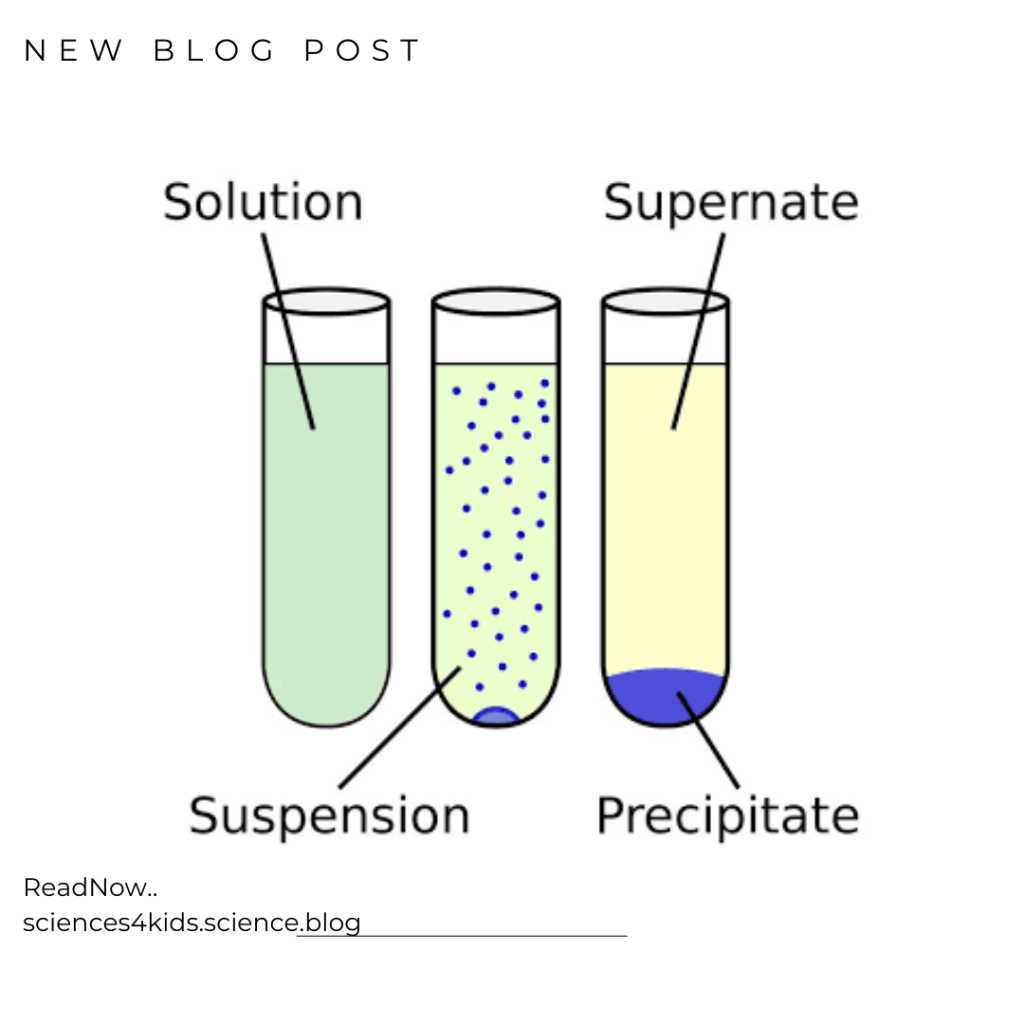
Precipitation is a separation technique that is used to separate substances based on their solubility. When two substances are mixed together, they can form a solid (called a precipitate) if they are not soluble in the same solvent. Precipitation takes advantage of this by mixing two substances together and then separating the precipitate from the mixture.
There are two main types of precipitation: neutralization and complexation.
Neutralization
Neutralization is used to separate an acid and a base. Acids and bases are special types of substances that have certain properties. When an acid and a base are mixed together, they form a neutral substance and a salt. The neutral substance and the salt can be separated from the mixture using precipitation.
Complexation
Complexation is used to separate metal ions. Metal ions are atoms that have a positive charge. They can form special bonds with other substances, called complexes. Complexation takes advantage of this by mixing a metal ion with a special substance that can form a complex with it. The complex can be separated from the mixture using precipitation.
Precipitation is a really useful technique because it can be used to purify compounds. Sometimes, we need to remove impurities from a compound so that we can study it or use it. Precipitation is also used to remove contaminants. Contaminants are substances that are not supposed to be in a mixture, and they can sometimes be harmful. By using precipitation, we can remove contaminants from a mixture.
In conclusion, separation techniques are important tools used by scientists to separate different substances from each other. We have learned about several different types of separation techniques, including distillation, filtration, chromatography, centrifugation, extraction, and precipitation. Each of these techniques has its own unique principle and uses, and they are all important in a variety of fields.
Looking to the future, there is ongoing research and development in the field of separation techniques. Scientists are constantly finding new and improved methods for separating substances, and there is always room for innovation. For example, researchers are currently working on developing new materials for use in chromatography that will be more efficient and effective at separating mixtures.
Overall, separation techniques are an essential part of many scientific fields, and they will continue to play a vital role in the advancement of science. We hope that you have enjoyed learning about these techniques with Sciences4Kids, and that you will continue to explore the exciting world of science!
Leave a comment